Draw The Lewis Structure Of Hcn
Draw The Lewis Structure Of Hcn - #1 first draw a rough sketch. Web lewis dot structure can be used to get the basic idea of the structure. Your solution’s ready to go! Draw the lewis structure for the hcn molecule. #3 calculate and mark formal charges on the atoms, if required. Because of the 2 pi bonds and 1 sigma bond formed by the hybridization of 2p x , 2p y , and 2p z between c and n atoms, this 2p overlap makes the bond stronger and shorter therefore the bond between c and n is linear.
For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Draw lewis structures depicting the bonding in simple molecules #1 first draw a rough sketch. Your solution’s ready to go! #2 mark lone pairs on the atoms.
Include all lone pairs of electrons. Organic chemistry lewis structures and bonding lewis dot diagram. Web the lewis structure (lewis dot diagram) for hcn.1. #4 convert lone pairs of the atoms, and minimize formal charges. For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule.
Draw the lewis structure for the hcn molecule. Web though we have seen the lewis structure of hcn we need to see how the 3d representation of the compound looks like. Web lewis dot structure can be used to get the basic idea of the structure. Web hey guys!in this video, we will look at the lewis structure of hydrogen.
The easiest way to find the molecular geometry of any compound is with the help of the vsepr theory. Lewis structures show all of the valence electrons in an atom or molecule. Post any question and get expert help quickly. Count the valence electrons you can use. Add these electrons to give every atom an octet.
A lewis structure is a way to show how atoms share electrons when they form a molecule. Here's how to do it. Draw lewis structures depicting the bonding in simple molecules And to find that we need to find the molecular geometry of the compound. Include all lone pairs of electrons.
Web drawing the lewis structure for hcn. The molecule is made up of one hydrogen ato. 288k views 10 years ago lewis structures practice problems with answers. Web write lewis symbols for neutral atoms and ions; Count the valence electrons you can use.
Count the valence electrons you can use. Add these electrons to give every atom an octet. To draw lewis structures for molecules and polyatomic ions with one central atom. If it is a polyatomic ion, like sulfate or nitrate, usually you put the heavy atom, or the atom to the left in the periodic table, in the center. In this.
Here, the given molecule is hcn. Put the least electronegative atom c in the middle with h and cl on either side. Web does this molecule exhibit resonance? Include all lone pairs of electrons. H + c + n =1 + 4 + 5 = 10.
And to find that we need to find the molecular geometry of the compound. There is a formal negative charge associated with this anion. Web your instructions for only $21/task. #3 calculate and mark formal charges on the atoms, if required. #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure.
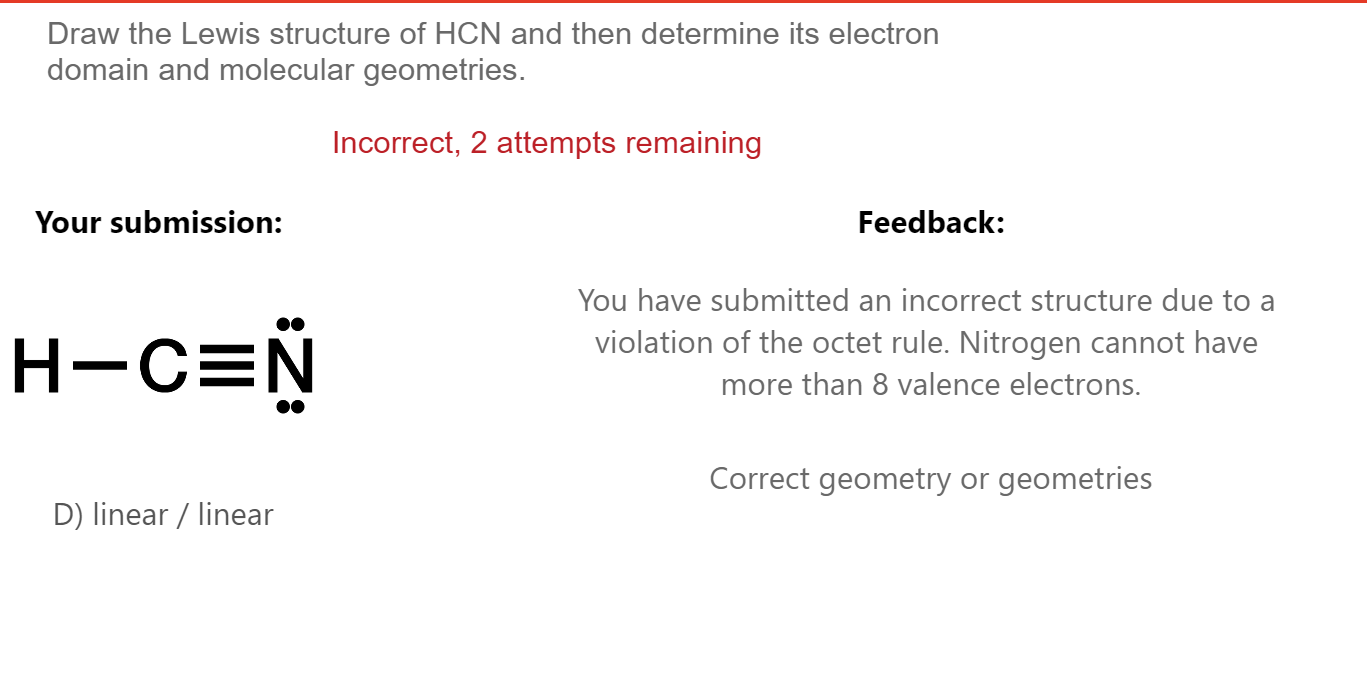
H + c + n =1 + 4 + 5 = 10. #4 convert lone pairs of the atoms, and minimize formal charges. Draw the lewis structure hcn and then determine its electron domain and molecular geometries. We'll also compare hnc to hcn and discuss why both are of inter. Your solution’s ready to go!
And to find that we need to find the molecular geometry of the compound. Draw the lewis structure for the hcn molecule. #1 first draw a rough sketch. H + c + n =1 + 4 + 5 = 10. 288k views 10 years ago lewis structures practice problems with answers.
Answer to draw the lewis structure for hcn. Organic chemistry lewis structures and bonding lewis dot diagram. Web write lewis symbols for neutral atoms and ions; Web use these steps to correctly draw the hcn lewis structure: #1 first draw a rough sketch.
Draw The Lewis Structure Of Hcn - #1 first draw a rough sketch. 6 steps to draw the lewis structure of hcn. Lewis structures show all of the valence electrons in an atom or molecule. Draw the lewis structure hcn and then determine its electron domain and molecular geometries. Count the valence electrons you can use. Put least electronegative atom in centre3. Web hey guys!in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. Here, the given molecule is hcn. Web the lewis structure (lewis dot diagram) for hcn.1. 14k views 10 years ago.
Count the valence electrons you can use. Your solution’s ready to go! In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. #2 mark lone pairs on the atoms. If it is a polyatomic ion, like sulfate or nitrate, usually you put the heavy atom, or the atom to the left in the periodic table, in the center.
H + c + n =1 + 4 + 5 = 10. #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure. You nave to put a triple bond between c and n. Put least electronegative atom in centre3.
#4 convert lone pairs of the atoms, and minimize formal charges. Count the valence electrons you can use. H + c + n =1 + 4 + 5 = 10.
Put the least electronegative atom c in the middle with h and cl on either side. With the lewis structure for hcn you’ll need to share more than one pair of electrons between the carbon and the nitrogen atoms. Be sure that you don't use more than the ten valence electrons available.
4K Views 6 Years Ago.
288k views 10 years ago lewis structures practice problems with answers. Web write lewis symbols for neutral atoms and ions; 6 steps to draw the lewis structure of hcn. 14k views 10 years ago.
In Order To Draw The Lewis Structure Of Hcn, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Hcn Molecule.
Here is a little flow chart of how we are going to do this: Put least electronegative atom in centre3. #3 calculate and mark formal charges on the atoms, if required. Here, the given molecule is hcn.
Include All Lone Pairs Of Electrons.
Here's how to do it. The lewis structure of hcn shows the arrangement of atoms and electrons in the molecule. The easiest way to find the molecular geometry of any compound is with the help of the vsepr theory. #4 convert lone pairs of the atoms, and minimize formal charges.
#5 Repeat Step 4 If Needed, Until All Charges Are Minimized, To Get A Stable Lewis Structure.
Web does this molecule exhibit resonance? In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. The hcn molecule consists of three atoms: Web learn to draw the lewis structure of hcn & understand molecular geometry, shape, & polarity about the same by reading this article.