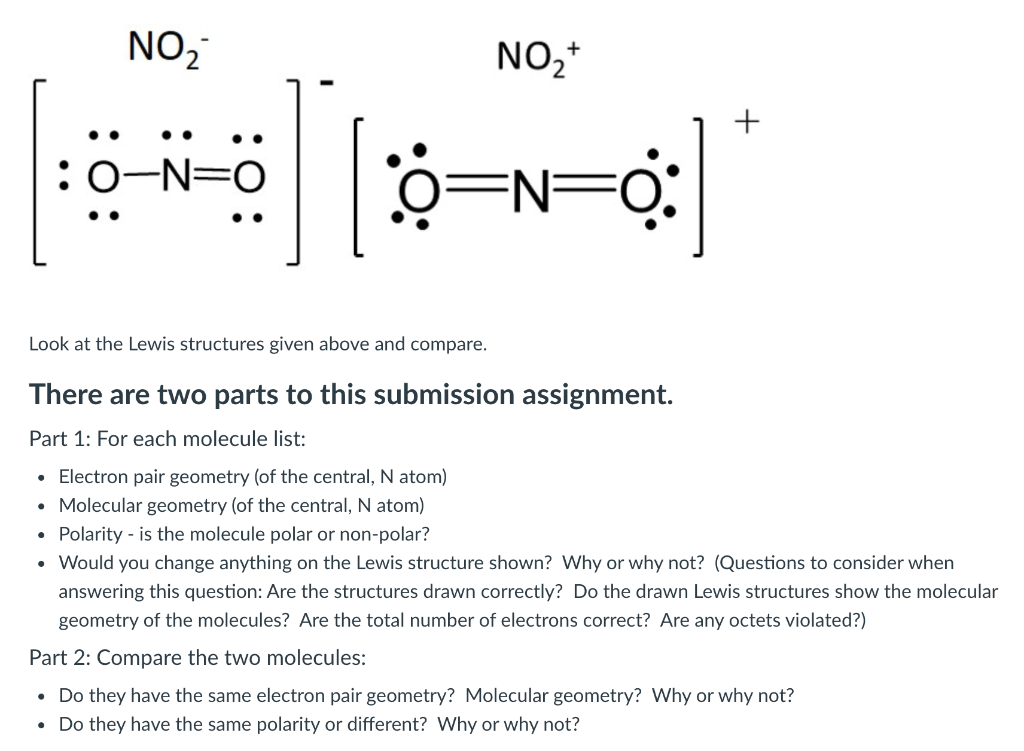
Draw The Lewis Structure For No2
Draw The Lewis Structure For No2 - There is 1 double bond and 1 single bond between the nitrogen atom (n) and each oxygen atom (o). Web let us talk about drawing the lewis structure for nitrogen dioxide ( no2 ). To learn about lewis structures, we will start with the lewis symbol. A lewis structure is a way to show how atoms share electrons when they form a molecule. Nitrogen will only have 7 valence electrons but this is the best we can do with this lewis structure. The no2 lewis structure has a total of 17 valence electrons.
There is 1 double bond and 1 single bond between the nitrogen atom (n) and each oxygen atom (o). Web lewis structure of no 2 (nitrogen dioxide) is drawn in this tutorial step by step. It is helpful if you: A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. This is a special case of lewis structure drawing because, there is.
This is a special case of lewis structure drawing because, there is. Connect the nitrogen atom to two oxygen atoms using single bonds. It is helpful if you: Web this chemistry video tutorial explains how to draw the lewis structure of no2 also known as nitrogen dioxide.how to draw lewis structures: To learn about lewis structures, we will start with the lewis symbol.
Try structures similar to no 2 for more practice. The no2 lewis structure has a total of 17 valence electrons. Web this chemistry video tutorial explains how to draw the lewis structure of no2 also known as nitrogen dioxide.how to draw lewis structures: For the no+ structure use the periodic table to find the total number of valence electrons for.
Lewis structures show all of the valence electrons in an atom or molecule. You can learn basics of how to draw a lewis structure properly from this example. Calculate the total number of valence electrons. To draw lewis structures for molecules and polyatomic ions with one central atom. 1.2.1 lewis structure of diatomic molecules.
Nitrogen belongs to group 15( or group 5) and has an atomic number of 7, therefore has a valency of 5. 1.2.1 lewis structure of diatomic molecules. A lewis structure, also known as an electron dot structure, is a simplified representation of a molecule's valence shell electrons. Try structures similar to no 2 for more practice. A lewis structure is.
For the no+ structure use the periodic table to find the total number of valence electrons for the no+. Web how do you draw the lewis structure of no2? 35k views 2 years ago lewis structures. Web let us talk about drawing the lewis structure for nitrogen dioxide ( no2 ). There is 1 double bond and 1 single bond.
Nitrogen will only have 7 valence electrons but this is the best we can do with this lewis structure. To draw lewis structures for molecules and polyatomic ions with one central atom. A lewis structure, also known as an electron dot structure, is a simplified representation of a molecule's valence shell electrons. There is 1 double bond and 1 single.
Try to draw the no 2 lewis structure before watching the video. Connect the nitrogen atom to two oxygen atoms using single bonds. Web lewis structure of no 2 (nitrogen dioxide) is drawn in this tutorial step by step. To learn about lewis structures, we will start with the lewis symbol. Calculate the total number of valence electrons.
The no2 lewis structure has a total of 17 valence electrons. Try structures similar to no 2 for more practice. Web to begin drawing the no 2 lewis structure, it is recommended to follow a systematic approach. It describes the arrangement of valence electrons around particular atoms in a molecule. Try to draw the no 2 lewis structure before watching.
Web this chemistry video tutorial explains how to draw the lewis structure of no2 also known as nitrogen dioxide.how to draw lewis structures: Then, identify the positions of the lone pairs on the atoms and assign formal charges, if required. Web lewis structure of no 2 (nitrogen dioxide) is drawn in this tutorial step by step. Web how do you.
To satisfy the octet on nitrogen, exactly one of the. Web how do you draw the lewis structure of no2? Then, identify the positions of the lone pairs on the atoms and assign formal charges, if required. First, sketch a rough skeleton structure of the molecule. 1.2.1 lewis structure of diatomic molecules.
Nitrogen will only have 7 valence electrons but this is the best we can do with this lewis structure. Find total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of. Connect the nitrogen atom to two oxygen atoms using single bonds. In order to draw the lewis structure of no2, first of all you.
Draw The Lewis Structure For No2 - 1.2.1 lewis structure of diatomic molecules. Web drawing the lewis structure for no 2 (nitrogen dioxide) the lewis structure for no 2 requires you to place fewer than 8 valence electrons on nitrogen (n). For the no+ structure use the periodic table to find the total number of valence electrons for the no+. A lewis structure, also known as an electron dot structure, is a simplified representation of a molecule's valence shell electrons. For the no2 structure use the periodic table to find the total number of valence electrons for the. To satisfy the octet on nitrogen, exactly one of the. Web how to draw the lewis dot structure for no2: To learn about lewis structures, we will start with the lewis symbol. Web how do you draw the lewis structure for no 2? Find total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of.
Calculate the total number of valence electrons. Here, the given molecule is no2 (nitrogen dioxide). Nitrogen belongs to group 15( or group 5) and has an atomic number of 7, therefore has a valency of 5. The no2 lewis structure has a total of 17 valence electrons. Try to draw the no 2 lewis structure before watching the video.
For the no2 structure use the periodic table to find the total number of valence electrons for the. To draw the lewis structure of no2 (nitrogen dioxide), start by placing the nitrogen atom in the center. Find total number of electrons of the valance shells of nitrogen and oxygen atoms and charge of. To learn about lewis structures, we will start with the lewis symbol.
In order to draw the lewis structure of no2, first of all you have to find the total number of valence electrons present in the no2 molecule. Connect the nitrogen atom to two oxygen atoms using single bonds. Watch the video and see if you missed any steps or information.
Try structures similar to no 2 for more practice. Nitrogen will only have 7 valence electrons but this is the best we can do with this lewis structure. It describes the arrangement of valence electrons around particular atoms in a molecule.
Web 5 Steps To Draw The Lewis Structure Of No2 Step #1:
Here, the given molecule is no2 (nitrogen dioxide). It is helpful if you: Connect the nitrogen atom to two oxygen atoms using single bonds. This is a special case of lewis structure drawing because, there is.
Find Total Number Of Electrons Of The Valance Shells Of Nitrogen And Oxygen Atoms And Charge Of.
Then, identify the positions of the lone pairs on the atoms and assign formal charges, if required. There is 1 double bond and 1 single bond between the nitrogen atom (n) and each oxygen atom (o). Web let us talk about drawing the lewis structure for nitrogen dioxide ( no2 ). A lewis structure, also known as an electron dot structure, is a simplified representation of a molecule's valence shell electrons.
35K Views 2 Years Ago Lewis Structures.
To draw lewis structures for molecules and polyatomic ions with one central atom. You can learn basics of how to draw a lewis structure properly from this example. Let us look at the periodic table. In order to draw the lewis structure of no2, first of all you have to find the total number of valence electrons present in the no2 molecule.
Web Drawing The Lewis Structure For No 2 (Nitrogen Dioxide) The Lewis Structure For No 2 Requires You To Place Fewer Than 8 Valence Electrons On Nitrogen (N).
Web how to draw the lewis dot structure for no2: For the no+ structure use the periodic table to find the total number of valence electrons for the no+. Web lewis structure of no 2 (nitrogen dioxide) is drawn in this tutorial step by step. Lewis structures show all of the valence electrons in an atom or molecule.